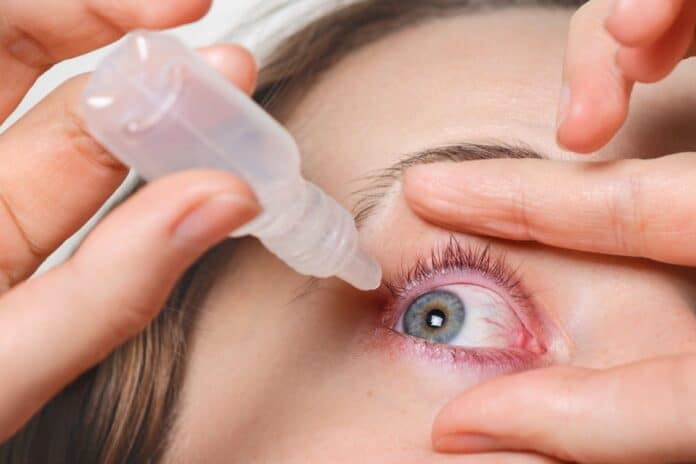
Eye drops have emerged as a promising solution for slowing down the progression of nearsightedness (myopia) in children. Traditional corrective measures like glasses and contact lenses provide immediate visual improvement but do not address the underlying issue of myopia advancement. Specialized eye drops, known as myopia control drops, contain substances designed to modify eye growth and reduce elongation.
Clinical trials have shown positive results, with significant reductions in axial elongation compared to control groups. Eye drops offer a non-invasive and convenient option, targeting the structural changes within the eye. Further research is needed, but the potential to slow myopia progression in children represents a significant advancement in pediatric eye care.
Promising results from a recent three-year clinical trial indicate that drug therapy for slowing the progression of nearsightedness in children may be on the horizon. The study found that daily low-dose atropine eye drops, known for pupil dilation, were more effective than a placebo in limiting changes in eyeglass prescriptions and inhibiting eye elongation in children aged 6 to 10 with nearsightedness.
Slowing down this elongation is crucial, as myopia worsens during the teenage years and can lead to various vision-related complications later in life. Most conventional corrective lenses do not address myopia progression, highlighting the significance of these findings.
A study led by Karla Zadnik from The Ohio State University has found that low-dose atropine solutions can significantly slow the progression of myopia in children. The study, called CHAMP (Childhood Atropine for Myopia Progression), assessed the safety and effectiveness of two low-dose atropine concentrations (.01% and .02%) compared to a placebo. Surprisingly, the .01% atropine solution showed the most significant improvements in slowing eye growth and reducing the need for stronger glasses prescriptions.
The trial involved 489 children aged 6 to 10 and demonstrated the safety and tolerability of the low-dose formulations. The experimental drug, manufactured by Vyluma, is being considered for approval by the FDA and could provide a future treatment option for the increasing prevalence of myopia worldwide.
The study’s results provide valuable insights into the potential benefits of low-dose atropine as a treatment for myopia, particularly in children. The experimental drug studied in the trial, manufactured by Vyluma, a biopharmaceutical company focusing on refractive errors of the eye, is being considered for approval by the FDA. It would be distributed in single-use packaging to ensure convenience and prevent contamination.
Furthermore, being the first to include placebo controls for three years and involving a large and diverse population recruited from multiple clinical sites, the CHAMP trial adds robustness to the research conducted on low-dose atropine. The ongoing evaluation of how the eyes respond after the treatment concludes will further enhance our understanding of this therapy’s long-term effects and benefits.
Overall, this study’s findings signify a significant advancement in myopia research and provide hope for developing effective treatments to address the rising rates of nearsightedness in children.